- 소개
- 학술지원 프로그램
소개
최고의 검사 품질을 바탕으로 고객맞춤형 R&D 지원 서비스를 제공합니다.
(재)씨젠의료재단은 2004년 R&D사업실을 창설하고,
축적된 연구의학 정보와 진단의학 전문 인력을 바탕으로 고품질 R&D 지원 서비스를 제공하고 있습니다.
축적된 연구의학 정보와 진단의학 전문 인력을 바탕으로 고품질 R&D 지원 서비스를 제공하고 있습니다.
특장점
01
석·박사 이상의연구개발 분야 전문인력 구성
02
연구기획 단계부터체계적인 컨설팅 서비스 제공
03
진단검사 분야 전문가의학술지원 서비스 제공
04
다년간 축적된 데이터베이스를 기반으로, 검체 수거부터 연구 및 결과 제공까지 Total Service 수행



서비스 절차

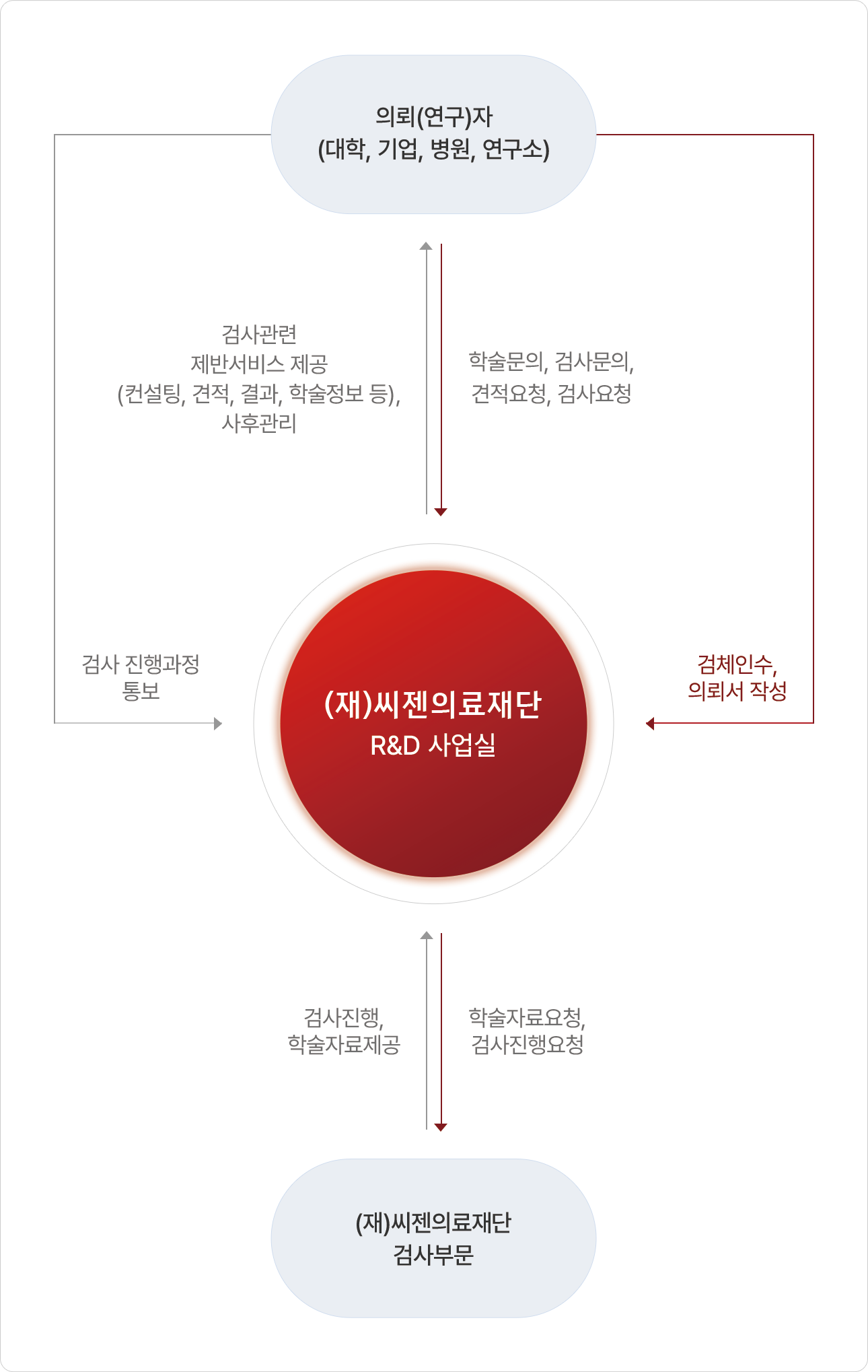
학술지원 프로그램
다양한 고객들의 연구 활동을 위한 R&D 학술지원 서비스를 실시합니다.학술지원 프로그램
진단검사 관련 최신 기술 및 전문인력을 바탕으로 공공기관 · 교육기관 및 연구소 · 의료기관 · 개인 등에게연구에 필요한 각종 임상검사 데이터 등을 제공합니다.
프로그램 항목
- 국가연구사업
- 임상검사사업, 검체자원화 사업, 전국 대단위 코호트 사업, 전국감시망 사업, 바이러스 질환 연구 사업, 감염병 진단관리 사업
- 환경보건연구사업
- 생체시료 중 유해물질 분석, 중금속 분석, 산화스트레스 바이오마커 분석, 대사체 분석, 환경호르몬 분석, 내분비 교란물질 분석
- 연구용 임상검사 분석
- 특수면역 ELISA 분석, 분자진단 검사(PCR · RT-PCR · NGS), 임상화학, 면역학, 핵의학 등
- 인체 유래물 자원제조
- 인체유래물 (혈청 · 혈장 · DNA · PBMC · 배양세포 · 조직 등) 제작
- 각종 생명연구 자원을 분리 및 보관
- 임상시험검체분석
- Central Lab. 임상시험
- 각종 임상시험에 필요한 임상검사 및 특수면역학 검사
- 개인연구용 임상검사 분석 지원
- 석 · 박사학위 분석자원
- 국 · 내외 학술 논문 발표 연구지원
- 인체 자원은행
- 국가인체 자원은행 시설관리 등에 준하는 체계 구축
- 다양한 생체시료를 적합한 조건에 보관, 시료의 입 · 출고 관리
- 24시간(무정전시스템) 보관 관리
- 실시간 온 · 습도 모니터링 구축
- 고품질검체운송사업
- 실시간 온도 · 위치 모니터링
- 검체별 특수전용검체운송 박스 자체제작 운용



















